Research
Our group research interest lies in the area of development of new methodologies for organic transformations, chiral catalyst design, and total synthesis of natural products and biologically relevant molecules.
C–C Scission Chemistry
Dealkenylative Synthesis
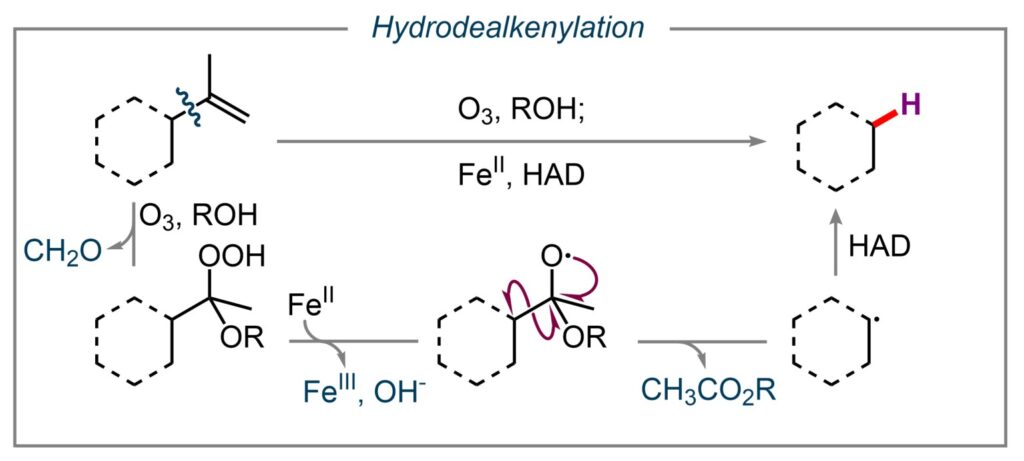
A recent focus in our laboratory is the activation of alkene C(sp3)–C(sp2) bonds, commonly found in abundant plant-based terpene and terpene-derived natural products. (1) These methodologies have applications in total synthesis and the rapid generation of biologically relevant molecules. Future efforts include developing novel variations of this methodology and designing sustainable catalytic systems for these transformations. (2, 3, 4, 5, 6) View our list of substrate alkenes here.
Deacylative Synthesis
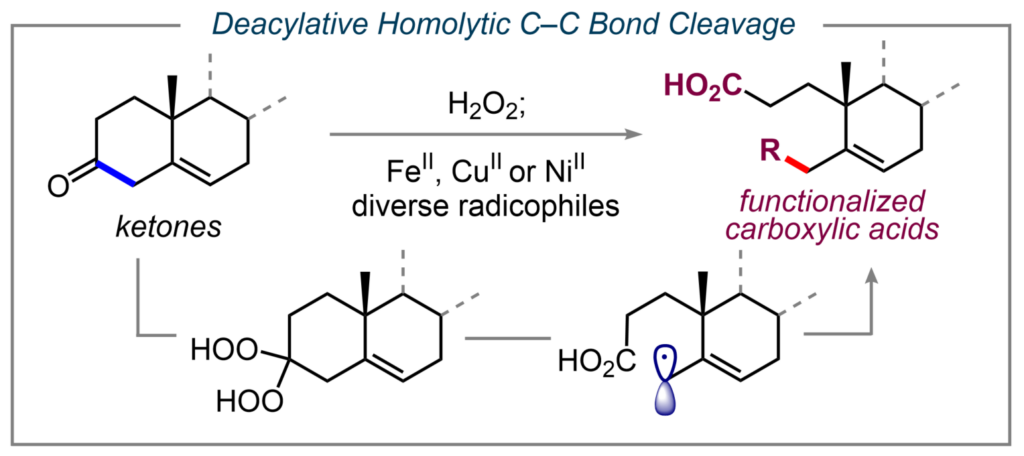
A similar strategy has been applied to homolytically cleave aliphatic ketones of various complexities. This allows for the transformation of cycloalkanones into carboxylic acids tethered to C-centered free radicals that can be engaged in diverse radical-based processes. High tolerance toward various functional groups and structures allows this method to reconstruct diverse ketone substrates, including chiral pool molecules, providing access to synthetically useful compounds. (7)
Phosphorous Organocatalysis
One of the central themes of research in our laboratories is the design and development of new reactions for enantioselective phosphine
catalysis and their applications in the chemical syntheses of natural products and unnatural small molecules of medicinal significance. Over the past twenty years, the lab has developed over 30 new phosphine-catalyzed reactions and helped pioneer the field of phosphine catalysis research.
Nucleophilic Phosphine Catalysis
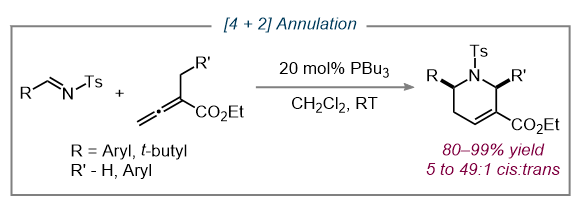
Nucleophilic phosphine catalysis reactions involve the conjoinment of two different substrates for assembling a large variety of molecular frameworks, including carbocycles and heterocycles. The first report of a phosphine-catalyzed [4 + 2] reaction came in 2003 from our lab, where annulations between α-alkylallenoates and N-tosylaldimines formed tetrahydropyridine products (8). This exciting discovery confirmed the versatility of phosphine-catalyzed annulations and initiated a new subclass of reactions.
Catalyst Design
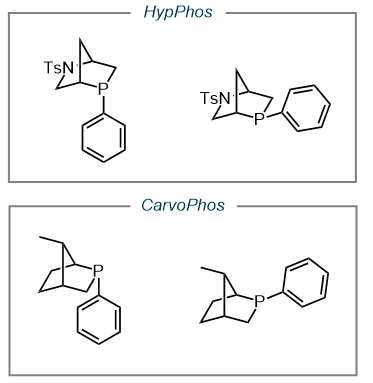
Our group developed a new family of L-hydroxyproline (Hyp)-derived [2.2.1] bicyclic phosphine catalysts, currently available from Sigma-Aldrich. These HypPhos catalysts have shown great synthetic utility, as demonstrated in multiple publications (9, 10), and proven the catalytic potential of the rigid [2.2.1] bicyclic framework. We have also developed a family of catalysts derived from R-(–)-Carvone, thus named CarvoPhos. (11)
Natural Product Total Synthesis
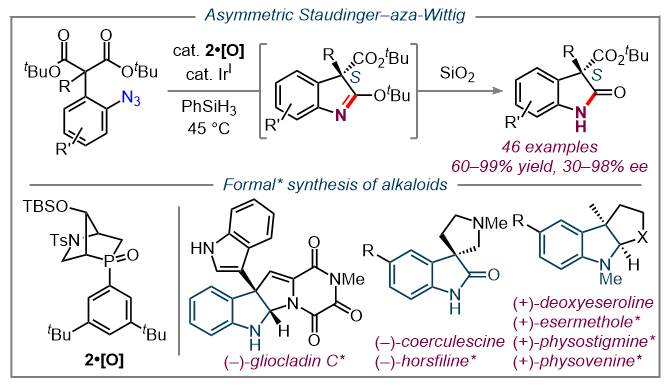
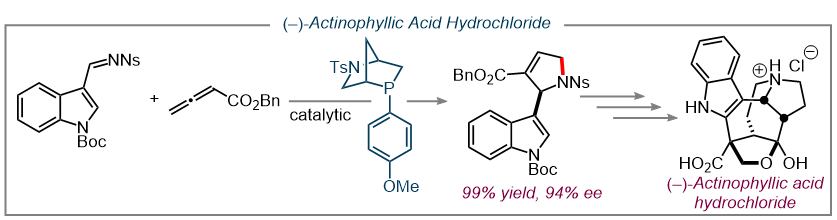
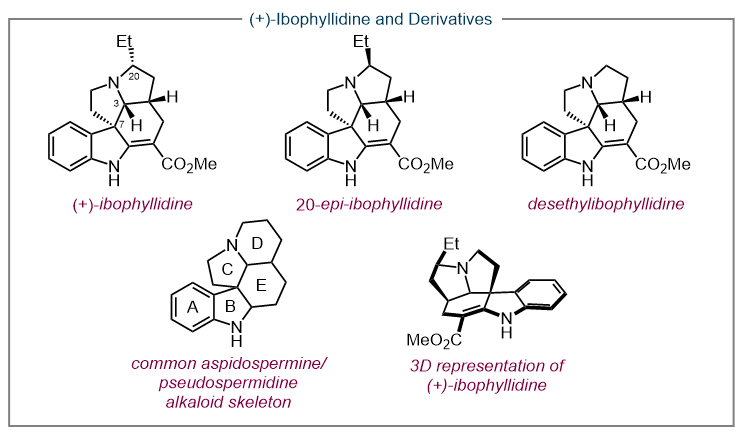
As a direct application of our methodologies, our lab has pursued a number of total syntheses of biologically significant natural products including (–)-Actinophyllic acid and (+)-ibophyllidine. (14, 15) These targets were both accessed via HypPhos-catalyzed [ 3 + 2 ] annulations.
Chemical Biology Applications
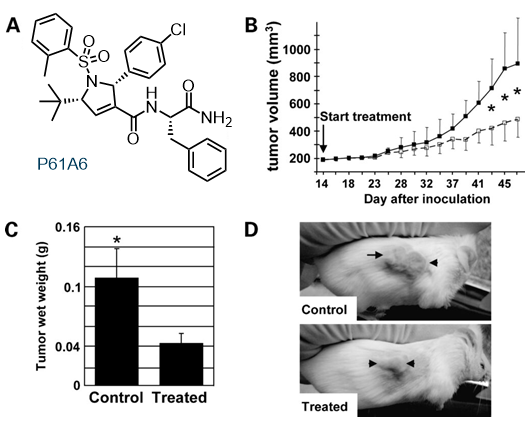
Collaboration with biologists has resulted in the discovery of several enzyme inhibitors, some of which show strong anti-tumor activity. (16, 17) Another small molecule, dubbed “efsevin,” is a potent modulator of cardiac rhythmicity through regulation of mitochondrial Ca2+ uptake via the voltage-dependent anion channel 2 (VDAC2). (18) It also displays prophylactic activity against ventricular tachycardia in a rodent model of a human heart disease. (19)
Nitrodiene Pericyclic Reactions
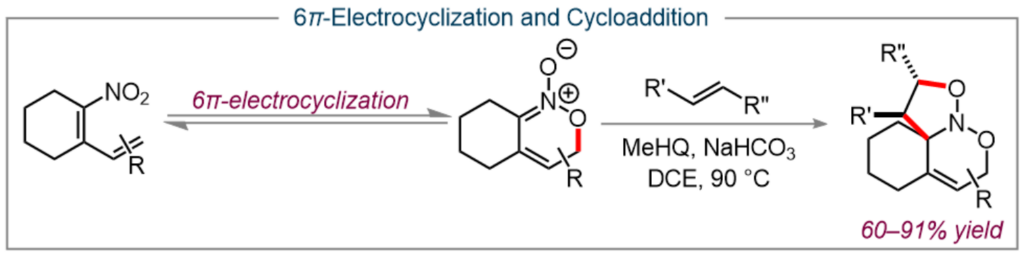
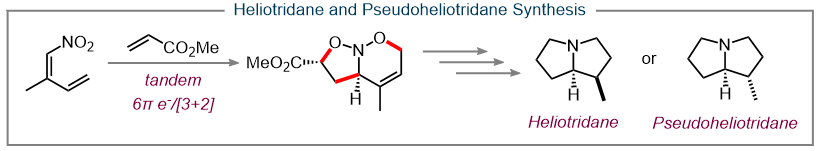
Another area of research in our lab has been the exploration of pericyclic reactions involving nitrodiene moieties. This includes a tandem 6π-electrocyclization and cycloaddition for the formation of multicyclic nitroso acetals which can then be leveraged for the total synthesis of of heliotridane and pseudoheliotridane. (20, 21)